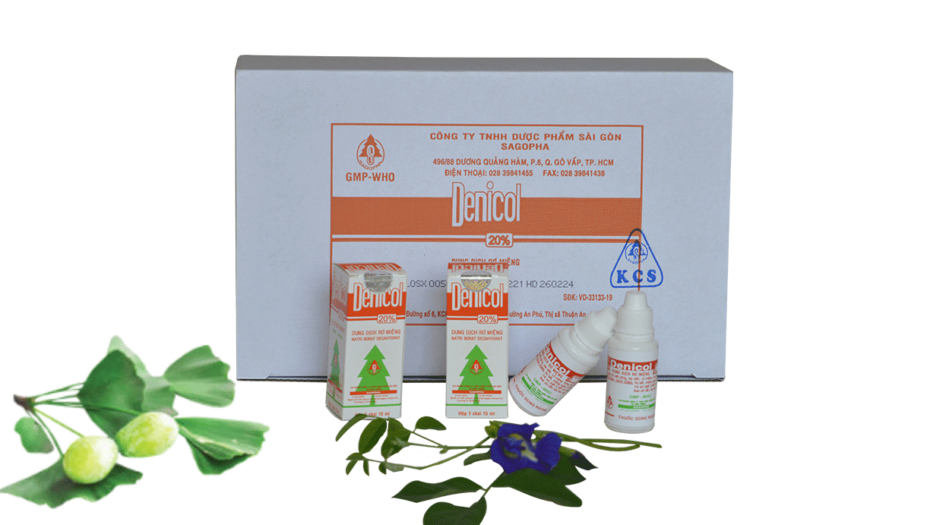
DENICOL
GENERAL INFORMATION
- DOSAGE FORM: Oral solution
-
PACKAGING:
15 ml a bottle with minibox
Totally 40 miniboxes a box
**Bottle by PP plastic. - STORAGE CONDITION: Dry place, protect the light, temperature not exceed 30 °C
- SHELF-LIFE: 36 months
- SPECIFICATION: Manufacturer's specification
ACTIVE INGREDIENT:
- Sodium borate (decahydrate) 3,87 g
- Glycerin, Vanilline q.s 15 mL
Product information is for reference only. Please see detailed drug information in the drug leaflet attached to the product.
INDICATIONS:
Treatment of oral thrush, mouth ulcer, inflammed gum.
CONTRAINDICATIONS:
Hypersensitivity to any ingredients of this product. Do not apply to inflamed mucosa.
USAGE INSTRUCTIONS AND DOSE:
- Apply a small amount on the tongue THREE times a day
- Do not use in children under 2 years of age unless advised by the doctor
WARNINGS AND CAUTIONS:
- Medications for external use, not to be taken.
- Avoid direct contact with eyes.
- Do not apply repeatedly over a large mucosal area.
- Stop taking the medicine and consult a doctor if symptoms persist and are severe.
ADVERSE DRUG REACTIONS:
No adverse drug reactions have been reported.
Inform your doctor and pharmacist of any adverse reactions encountered while using the drug.
WARNINGS FOR THE USE OF DRUGS DURING PREGNANCY AND BREASTFEEDING:
- Use of drugs for pregnant women
- There are no data on the use of the drug in pregnant women, avoid use in pregnant women.
- Use of drugs for breastfeeding women
- There are no data on the use of the drug in nursing women, should only be used if the benefits outweigh the risks.
DRUG INTERACTIONS AND INCOMPATIBILITIES:
- Interactions: Do not have any study about interaction
- Incompatibilities: There is no study on drug incompatibilities, do not mix this drug with other drugs
OVERDOSE AND TREATMENT:
- Overdose: There are no data on overdose, do not exceed the indicated dose of the drug
- How to handle drug overdose: Actively monitor to take timely measures


 EN
EN VN
VN