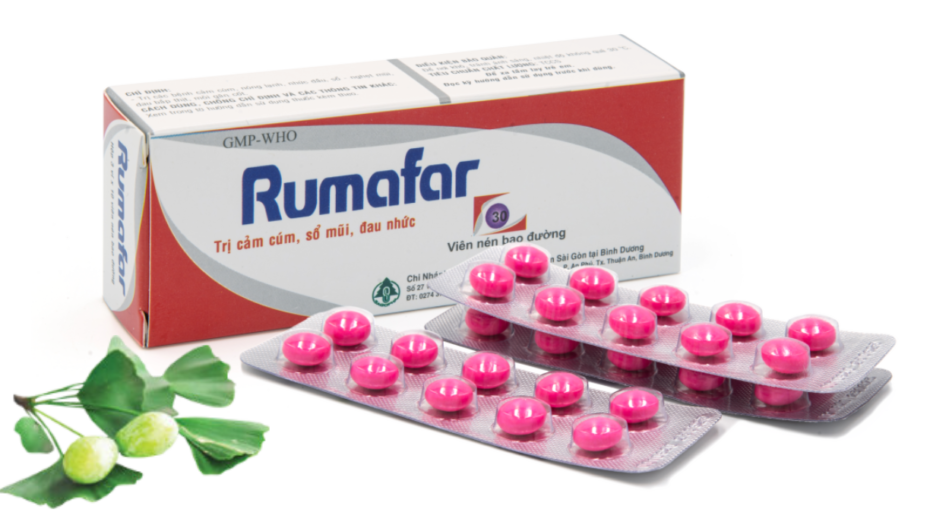
Rumafar
GENERAL INFORMATION
- DOSAGE FORM: Sugar-coated tablets
- PACKAGING: Box of 3 blisters; 10 tablets a blister
- STORAGE CONDITIONS: Dry place, protect the light, temperature not exceed 30 °C
- SHELF-LIFE: 36 months
- SPECIFICATION: Manufacturer's specification
ACTIVE INGREDIENT:
- Radix Plucheae pteropodae extract 150 mg
- Rhizoma Ligustici watlichii extract 50mg
- Dry powder of Fructus Viticis trifoliae 100 mg
- Dry powder of Radix Angelicae dahuricae 50 mg
- Dry powder of Rhizoma Kaempferiae galangae 30 mg
- Dry powder of Alumen 10 mg
Product information is for reference only. Please see detailed drug information in the drug leaflet attached to the product.
INDICATIONS:
Assists in relieving cold and flu symptoms such as stuffy noses and muscles aches.
CONTRAINDICATIONS:
- Hypersensitivity to any ingredients of this product.
- Pregnant women
- People with diabetes
- Children under 5 years old
- Children have medical history of seizure or febrile seizure.
USAGE INSTRUCTIONS AND DOSE:
- Children:
- 5-9 years of age: take 1 tablet THREE times a day
- 10-15 years of age: take 1-2 tablet(s) THREE times a day
- Adult:
- Take 2 tablets Three to Four times a day
WARNINGS AND CAUTIONS:
There are no data on warnings and precautions for use.
ADVERSE DRUG REACTIONS:
No adverse drug reactions have been reported.
Inform your doctor and pharmacist of any adverse reactions encountered while using the drug.
WARNINGS FOR THE USE OF DRUGS DURING PREGNANCY AND BREASTFEEDING:
Pregnant women
DRUG INTERACTIONS AND INCOMPATIBILITIES:
Drug interactions: There have been no drug interaction studies, do not take this drug with other drugs.
Drug incompatibilities: Since there have been no studies on drug incompatibility, this drug should not be mixed with other drugs.
OVERDOSE AND TREATMENT:
Overdose: There are no data on overdose, do not exceed the indicated dose of the drug.
How to deal with drug overdose: Actively monitor for timely handling measures.


 EN
EN VN
VN